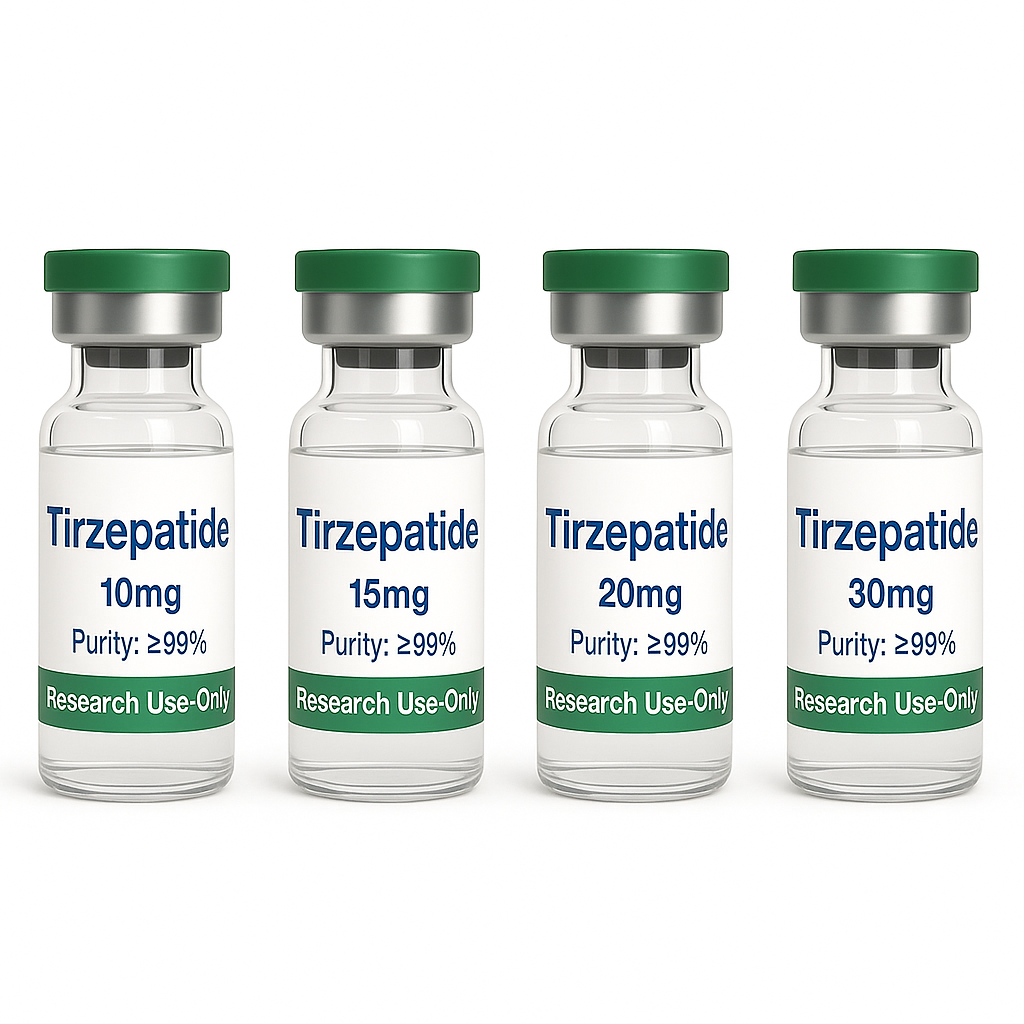
I-Tirzepatide Lyophilized High Purity 99% Peptide Powder TR 10mg 15mg 20mg 30mg ibhodlela ngalinye likashukela/isifo sikashukela
Imininingwane Yomkhiqizo
| Igama | I-Tirzepatide Injection Powder |
| Ubumsulwa | 99% |
| Ukubukeka | Impushana Emhlophe Ye-Lyophilized |
| Ukuphatha | Umjovo we-Subcutaneous |
| Usayizi | 10mg, 15mg, 20mg, 30mg |
| Amanzi | 3.0% |
| Izinzuzo | Ukwelapha isifo sikashukela, ngcono ukulawula ushukela egazini |
Incazelo
I-Tirzepatide iyinoveli encike kushukela we-insulinotropic polypeptide/glucagon-like peptide 1 (GLP-1) receptor agonist egunyazwe e-United States njengesengezo ekudleni nasekuzivocavoceni ukuze kuthuthukiswe ukulawulwa kwe-glycemic kubantu abadala abanesifo sikashukela sohlobo 2 futhi ngaphansi kophenyo ukuze isetshenziswe ekulawulweni kwesisindo esingamahlalakhona, izenzakalo ezinkulu ezimbi zenhliziyo nemithambo yegazi kanye nokuphathwa kokwehluleka kwenhliziyo nezinye izimo zokukhuluphala, non-cirrhotic non-alcohol steatohepatitis. Uhlelo lweSigaba 3 SURPASS 1-5 lwesivivinyo somtholampilo lwaklanyelwe ukuhlola ukusebenza kahle nokuphepha kwe-tirzepatide ejovwe kanye ngesonto ngaphansi kwesikhumba (5, 10 kanye no-15 mg), njenge-monotherapy noma ukwelashwa okuhlangene, kubantu abaningi abanesifo sikashukela sohlobo 2. Ukusetshenziswa kwe-tirzepatide ezifundweni zomtholampilo kwakuhlotshaniswa nokwehla okuphawulekayo kwe-hemoglobin ye-glycated (-1.87 kuya ku-2.59%, -20 kuya ku-28 mmol/mol) nesisindo somzimba (-6.2 kuya ku-12.9 kg), kanye nokuncipha kwemingcele evame ukuhlotshaniswa nokwanda kwengozi ye-cardiometabolic njengomfutho wegazi, i-visceride circulatory and triglycerides. I-Tirzepatide yabekezelelwa kahle, ngengozi ephansi ye-hypoglycemia lapho isetshenziswa ngaphandle kwe-insulin noma i-insulin secretagogue futhi yabonisa iphrofayili yokuphepha ejwayelekile efana nesigaba se-GLP-1 receptor agonist. Ngakho-ke, ubufakazi obuvela kulezi zivivinyo zomtholampilo buphakamisa ukuthi i-tirzepatide inikeza ithuba elisha lokwehlisa ngempumelelo i-glycated hemoglobin nesisindo somzimba kubantu abadala abanesifo sikashukela sohlobo 2.